Electroplating is the deposition of a metallic coating onto an object by putting a negative charge onto the object and immersing it into a solution which contains a salt of the metal to be deposited. The metallic ions of the salt carry a positive charge and are attracted to the part. When they reach it, the negatively charged part provides the electrons to “reduce” the positively charged ions to metallic form.
What is Electroplating?
How does it work?
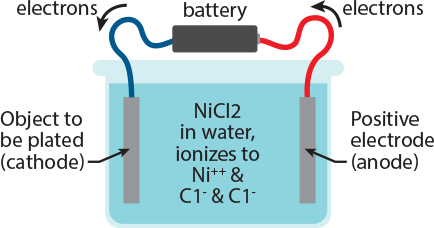
Look at the figure to the right, and then follow the written explanation. electroplating Imagine that we have an object that is made of copper or steel, and that it has been properly cleaned, and that we now want to plate it with nickel. A wire is attached to the object, and the other end of the wire is attached to the negative pole of a battery (the wire is blue in this picture). To the positive pole of the battery we connect the red wire; the other end of the red wire we connect to a rod made of nickel. Now we fill the cell with a solution of a salt of the metal to be plated. Because the object to be plated is negatively charged, it attracts the positively charged Ni++. The Ni++ ions reach the object, and electrons flow from the object to the Ni++. For each atom of Ni++, 2 electrons are required to neutralize it or ‘reduce’ it to metallic form. At the anode, electrons are removed from the Nickel metal, oxidizing it to the Ni++ state. Thus the nickel metal dissolves as Ni++ into the solution, supplying replacement nickel for that which has been plated out, and we retain a solution of nickel chloride in the cell.